Spectra
Click here to go to the
UPSCALE home page.
Click here to go
to the Physics Virtual Bookshelf.
Click here to
go to the JPU200Y home page.
This document is a brief introduction to atomic spectra at a
non-technical level. First, we will introduce and/or review some basic facts
about all waves, including light waves.
Waves
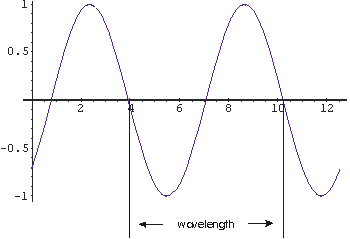 As proved by Thomas Young in the early nineteenth
century, light is a wave. Later in the century Faraday and Maxwell realised
that it is a wave of electric and magnetic fields, and now we often call it
electromagnetic radiation. The strength of the fields is called the
amplitude of the wave; in the figure to the right the amplitude is one.
As proved by Thomas Young in the early nineteenth
century, light is a wave. Later in the century Faraday and Maxwell realised
that it is a wave of electric and magnetic fields, and now we often call it
electromagnetic radiation. The strength of the fields is called the
amplitude of the wave; in the figure to the right the amplitude is one.
The wavelength of the wave is the length from a point on the wave
to the point where the oscillation begins to repeat; in the figure it is about
6.3 units.
The wavelength of electromagnetic radiation can range from very small to
very large. If the wavelength is between 400 and 700 billionths of a meter, our
eyes are sensitive to it and we call it light. The smaller wavelength we see as
the color violet; as the wavelength increases the we see color change to blue,
green, yellow, orange and red in that order. If the wavelength is out of this
range the physical phenomenon is the same but we give it different names such
as radio waves, X rays, gamma rays, etc.
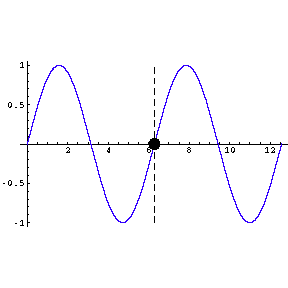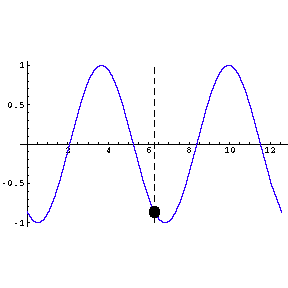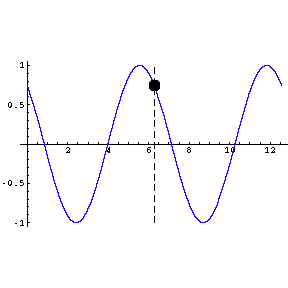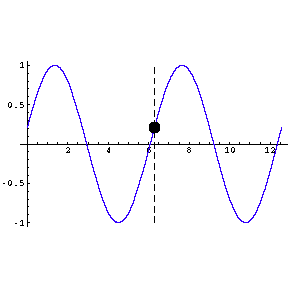 |
To the left we show a wave travelling through space. We also
indicate by means of a small circle the amplitude of the wave as it goes by us.
The frequency of oscillation of the dot is the frequency of the
wave.
The animation is a bit "chunky" to keep its total file size to a
reasonable 45k.
Technical note: the frequency of a wave times its wavelength is
equal to the speed of the wave. |
Spectra
|
Glasses tend to bend the path of light, a phenomenon called
refraction. For many glasses, the amount of bending depends on the
wavelength of the light; such glasses are called dispersive. Thus, if we
have white light incident on a prism made of a dispersive glass, we break the
light into its component colors. Newton was one of the first people to
investigate this "phenomenon of the colours." |
 |
|
If we look more carefully at the spectrum from the Sun, we see
that there are some discrete wavelengths that are missing. This is shown to the
right, and we will explain it soon.
If we have a trace amount of a pure element and subject it to a
strong electric field, it will emit light whose color is characteristic of the
particular element. Neon lights are an example of this phenomenon.
Some spectra of pure elements are shown to the right.
Note that for pure elements the spectra consists of discrete
wavelengths. In the early 1900's there were attempts to explain these line
spectra by analogy to the standing waves of organ pipes. A document on
standing waves is available
here.
The Sodium spectrum looks like a single wavelength, but is
actually two nearly identical wavelengths.
Note that there is some hint of a pattern in the wavelengths for
the Hydrogen spectrum. |
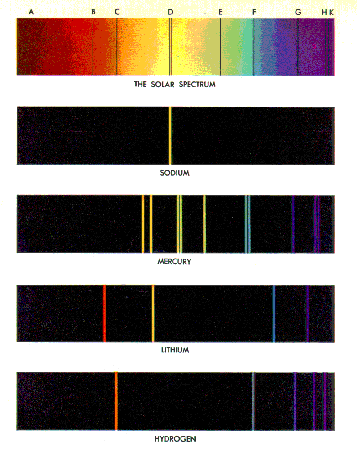 |
The spectrum of each pure element is unique, and in the late nineteenth
century the values of the line spectra for many elements were well known.
In the 1880's there was a German schoolteacher named Balmer whose hobby
was numerology. He was into trying to find significance in prime factors of the
number of steps of the great pyramid and things like that. He had a friend who
was a chemist, who suggested that he have a bash at the Hydrogen spectrum.
Through trial and error he came up with a simple formula. For completeness,
here is is:
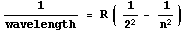
Here R is a constant equal to about 10,970,000 and n is
any integer greater than 2, such as 3, 4, 5, 108, etc.. Each different value of
n gives the wavelength of a different line in the Hydrogen spectrum.
Finally, we end by discussing the missing lines in the solar spectrum.
The wavelength of one of the lines in a pure element spectrum is fixed provided
the element is stationary relative to us. If it is moving, say, towards us the
Doppler effect will decrease its wavelength and increase its frequency;
if it is moving away the wavelength increases and the frequency decreases. This
is true for all waves, and you have probably noticed the effect when a car
playing loud music passes by you. For the elements in the gas lamps whose
spectra we showed above, the Doppler effect turns out to be negligible.
However, the temperature of the interior of the Sun is so high that the
atoms are vibrating very fast. The Doppler effect strongly smears out the
wavelengths of the lines from all the elements that are emitting the light.
Thus, the spectrum emitted by the interior of the Sun is continuous, not a
large collection of discrete wavelengths.
If an element has a particular spectrum, then the wavelengths of that
spectrum are also the wavelengths that the element can absorb from an incident
light beam. The outer mantle of the Sun is relatively cool, and the elements in
that mantle absorb wavelengths of the light from the interior corresponding to
their own light spectra. Thus, you can see that missing from the solar spectrum
are the lines corresponding to Sodium. From this we know that Sodium is present
in the outer mantle of the Sun.
In fact, the element Helium was first discovered by analysing missing
lines in the spectrum of the Sun.
This document is Copyright © 1999 David M. Harrison. This is
version 1.5, date (m/d/y) 01/01/00.
 As proved by Thomas Young in the early nineteenth
century, light is a wave. Later in the century Faraday and Maxwell realised
that it is a wave of electric and magnetic fields, and now we often call it
electromagnetic radiation. The strength of the fields is called the
amplitude of the wave; in the figure to the right the amplitude is one.
As proved by Thomas Young in the early nineteenth
century, light is a wave. Later in the century Faraday and Maxwell realised
that it is a wave of electric and magnetic fields, and now we often call it
electromagnetic radiation. The strength of the fields is called the
amplitude of the wave; in the figure to the right the amplitude is one.


