Subatomic Physics Detectors
Measure for every particle produced in an interaction.
|
|
|
|
|
|
|
|

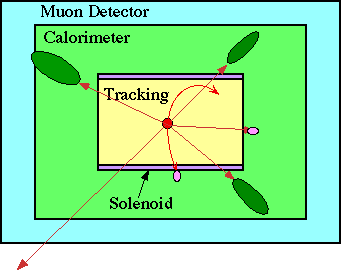
Tracking detectors measure the positions of charged particles.
A solenoid provides a magentic field so the momentum of charged particles can be determined from their radius of curvature.
Calorimeters measure the energy of hadrons, photons, and electrons.
Muon detectors identify muons because muons and neutrinos are the only particles which can pass through the the calorimeters, but muons ionize and neutrinos don't.
Measuring energy
Calorimeters measure energy by absorbing particles. Most calorimeters measure the ionization energy deposited by all the charged particles in the "showers" produced as the particle is absorbed. The scale length of electromagnetic showers is the radiation length, X0 (~2cm in Fe), relevant for both bremstrahlung pair production; the scale length for hadronic showers is the mean hadronic interaction length, lI (0.2m in Fe).
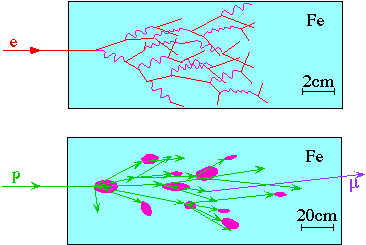
Ionization observed is proportional to energy lost
This is statistical process
The resolution degrades rapidly if all the particles are not absorbed. i.e. The fluctuations in how much energy leaks out can be much bigger than the statistical uncertainty on measuring the energy that does not leak out.
Photons from Natural Radioactivity observed with a Germanium semiconductor detector
The common natural radioactive isotopes are Potassium-40, Uranium-235, Uranium-238, and Thorium-232.
Natural potassium (Z=19 protons) has 3 isotopes (N=20, 21, or 22 neutrons):
Potassium-40 decays (from National Nuclear Data Center and Table of Nucleides):
 e e | |||
| 19K40 + e-atomic Þ 18Ar40*+ne
(18Ar40*Þ 18Ar40+g) |
|
Beta decay
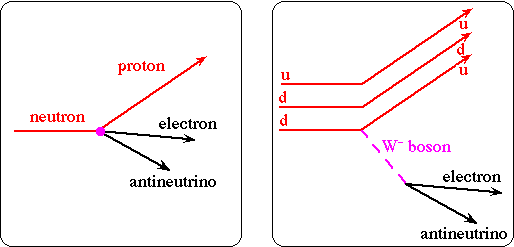
If particles can decay to a lower energy state, they will. Looking at some mass 40 isobars we can predict which isotopes will undergo beta decays:
atomic mass unit (u) = (mass 12C atom)/12 = 931.49432(28)MeV
A typical 70kg human body has 140g of potassium, and 17mg of K40, corresponding to a decay rate of 4.4kBq, and an annual dose of about 0.2mSv (out of a typical total of about 3.6mSv).
| SI Unit | Old Units | |
| Activity: | Bequerel (Bq) = 1 decay/s | Curie (Ci)= 3.7x1010 Bq |
| Absorbed Dose | Gray (Gy) = 1 Joule/Kg | Rad = 0.01 Gy |
| Equivalent Dose | Sievert (Sv) = Gray x Q
(Q = radiation weighting factor) | Rem = 0.01 Sv |
Photon total cross sections in Carbon (Z=6) and Lead (Z=82), showing the dominant contributions for carbon.
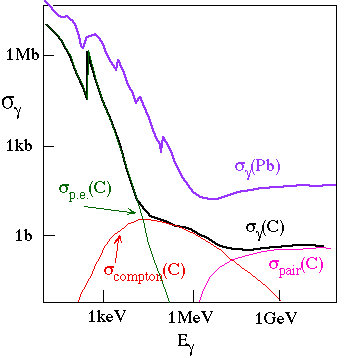
Real photons spend part of their time (about a) as a virtual electron-positron pair. These virtual e+e- pairs can scatter from the virtual photons in the electromagnetic field of a charged particle, e.g. an atomic nucleus or electron, and become a real e+e- pair.

In the high energy limit (Eg>>2me), the mean distance a photon will travel before pair producing is
(Note: The reason the radiation length and the pair production length are almost the same is because bremsstrahlung and pair production are simply time and space rearrangements of the same process: a real photon coupling to an electron which is coupled via a virtual photon to a nucleus.)